
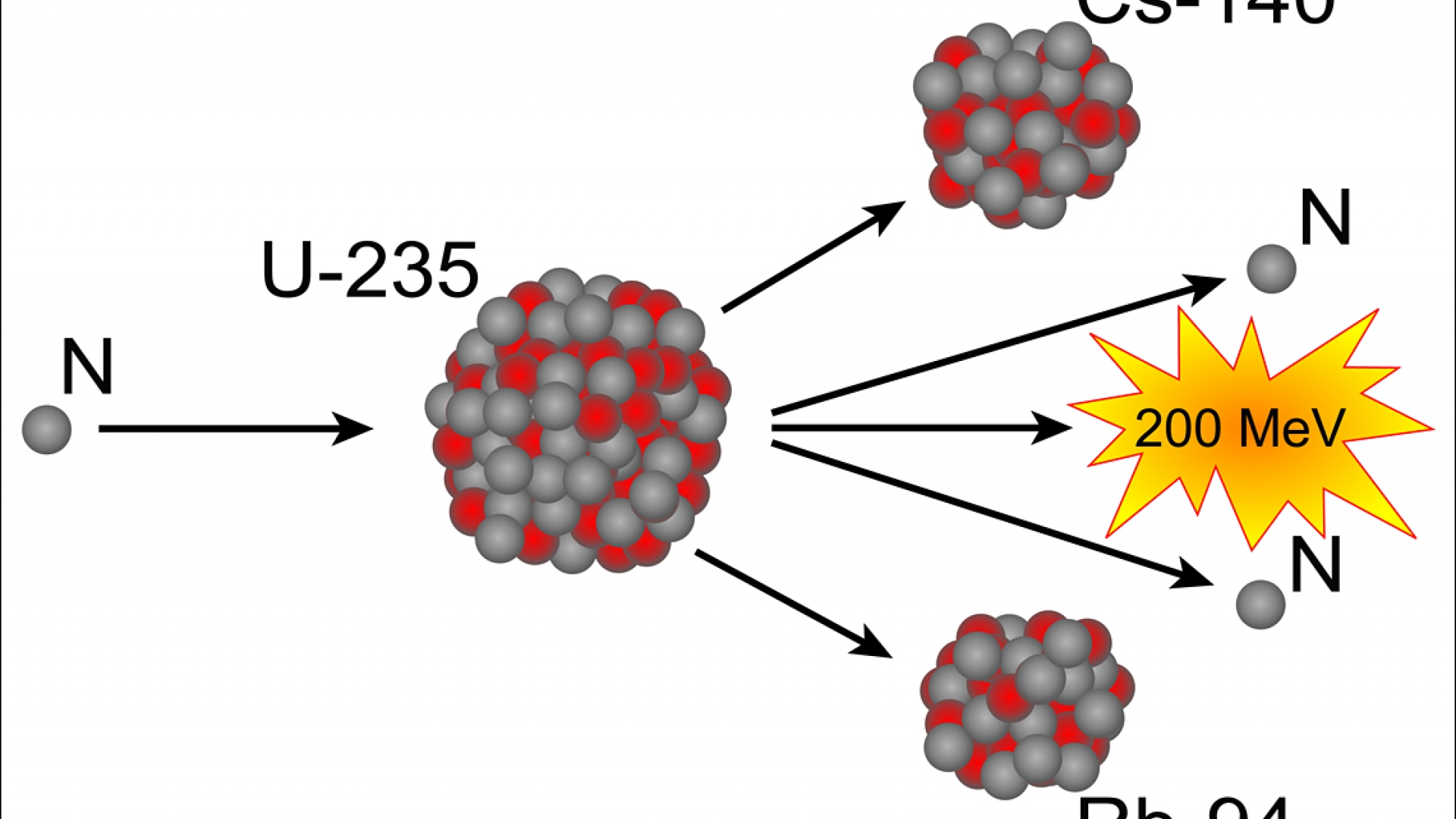
Ida Noddack suggested that instead of creating a new, heavier element 93, it was conceivable that the nucleus had broken up into large fragments, and Aristid von Grosse suggested that what Fermi's group had found was an isotope of protactinium. However, not everyone was convinced by Fermi's analysis of his results. Fermi won the 1938 Nobel Prize in Physics for his "demonstrations of the existence of new radioactive elements produced by neutron irradiation, and for his related discovery of nuclear reactions brought about by slow neutrons". Enrico Fermi and his colleagues in Rome studied the results of bombarding uranium with neutrons, and Fermi concluded that his experiments had created new elements with 93 and 94 protons, which his group dubbed ausenium and hesperium. The discovery of the neutron by James Chadwick in 1932 created a new means of nuclear transmutation. The discovery came after forty years of investigation into the nature and properties of radioactivity and radioactive substances. Hahn was awarded the 1944 Nobel Prize in Chemistry for the discovery. By analogy with the division of biological cells, he named the process "fission". Meitner calculated that the energy released by each disintegration was approximately 200 megaelectronvolts, and Frisch observed this. Meitner and her nephew Frisch theorised, and then proved, that the uranium nucleus had been split and published their findings in Nature. They reported their findings by mail to Meitner in Sweden, who a few months earlier had fled Nazi Germany. Hahn suggested a bursting of the nucleus, but he was unsure of what the physical basis for the results were. Hahn and Strassmann at the Kaiser Wilhelm Institute for Chemistry in Berlin bombarded uranium with slow neutrons and discovered that barium had been produced. Scientists already knew about alpha decay and beta decay, but fission assumed great importance because the discovery that a nuclear chain reaction was possible led to the development of nuclear power and nuclear weapons. The fission process often produces gamma rays and releases a very large amount of energy, even by the energetic standards of radioactive decay. Fission is a nuclear reaction or radioactive decay process in which the nucleus of an atom splits into two or more smaller, lighter nuclei and often other particles. Nuclear fission was discovered in December 1938 by chemists Otto Hahn and Fritz Strassmann and physicists Lise Meitner and Otto Robert Frisch. Three types of moderators are used at the MIT reactor: (1) ordinary or "light" water that is also used to cool the reactor core, (2) deuterated or heavy water (D 20), and (3) high-purity graphite, both of which are excellent at slowing neutrons without absorbing them.The nuclear reaction theorised by Meitner and Frisch. Since U-235 nuclei do not readily absorb the high energy neutrons that are emitted during fission, it is necessary to slow the neutrons down with a "moderator". In the MIT reactor, one other group of components is essential to the maintaining and controlling a chain reaction. As fewer and fewer neutrons are absorbed, more and more neutrons are available to cause the splitting of uranium nuclei, until finally enough neutrons are available to sustain a chain reaction. To put the reactor into operation, the control blades are raised very slowly. When the control blades are fully inserted, they absorb so many neutrons from the uranium that there are not enough to allow a chain reaction to continue. Boron has the property of absorbing neutrons without re-emitting any. The rate of fissions in the uranium nuclei in the MIT reactor is controlled chiefly by six control blades of boron-stainless steel which are inserted vertically alongside the fuel elements. When it is in operation, the central active core contains a huge number of neutrons traveling in every direction at very high speeds. The MIT Research Reactor is used primarily for the production of neutrons. Hence, the possibility exists for creating a chain reaction. Each time a U-235 nucleus splits, it releases two or three neutrons. This process is known as fission (see diagram below). When a U-235 nucleus absorbs an extra neutron, it quickly breaks into two parts. The arrangement of particles within uranium-235 is somewhat unstable and the nucleus can disintegrate if it is excited by an outside source. In the nucleus of each atom of uranium-235 (U-235) are 92 protons and 143 neutrons, for a total of 235.


 0 kommentar(er)
0 kommentar(er)
